 |
Science Frontiers ONLINE No. 53: Sep-Oct 1987 |
|
|
Has the second law been repealed?
"From the largest to the smallest scales, the universe is evolving. Matter, in the form of galaxies, is undergoing a colossal expansion. Gas, condensed into stars, is radiating thermonuclear energy out across an infall of matter, drawn by gravity. The simplest of chemical reactions and the most complex of biological activities are occurring on the surface of the earth in a state far from equilibrium; they are heated by the sun and cooled by the vacuum of space. This pervasive cosmic imbalance is the driving force in producing an environment conducive to the formation of structure and complexity."
This sweeping statement seems to apply to the entire universe. The Second Law of Thermodynamics, however, insists that, on the average, for the entire universe, the above paragraph cannot be true.
The article introduced by this unqualified assertion about the evolution of the universe is really about self-organizing chemical reactions. We classify it under biology because the authors imply that some biological phenomena are self-organizing.
The famous Belousov-Zhabotinskii reaction is used as the prime example of chemical self-organization. First, one takes a shallow dish filled with a solution of bromate ions in a highly acidic medium. Here's what happens:
"A dish, thinly spread with a lightly colored liquid, sits quietly for a moment after its preparation. The liquid is then suddenly swept by a spontaneous burst of colored centers of chemical activity. Each newly formed region creates expanding patterns of concentric, circular rings. These collide with neighboring waves but never penetrate. In some rare cases rotating one-, two-, or three-armed spirals may emerge. Each pattern grows, impinging on its neighboring patterns, winning on some fronts and losing on others, organizing the entire surface into a unique pattern. Finally, the patterns decay and the system dies, as secondary reactions drain the flow of the primary reaction."
From this starting point, the implication is made that all manner of biological "reactions" are analogous and therefore reducible to nought but physics and chemistry. Some examples given of self-organizing biological phenomena are: (1) the sequencing of amino acids into selfreplicating structures; (2) slime-mold organization; and (3) the origin of the lens structure of the firefly. All of these claims are accompanied by computer simulations of self-organizing reactions.
(Madore, Barry F., and Freedman, Wendy L.; "Self-Organizing Structures," American Scientist, 75:252, 1987.)
Comment. While we believe that science is the best way yet discovered to search for truth, we have to admit that scientists sometimes get carried away in their zeal to explain things, especially with computer graphics. The Belousov-Zha botinskii reaction is certainly impressive. So is crystal growth. But are the atoms falling together to form a crystal analogous to soldiers falling into ranks; or the assembly of genetic information into the genotypes for our planet's multitudinous species? How far can we apply reductionism?
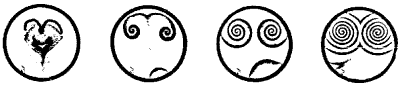 | Spectacular, evolving forms erupt in the Belousov-Zhabotinskii reaction. Waves of chemical activity propagate through a receptive liquid medium. |